NIH tech transfer office speeds global health solutions
March / April 2014 | Volume 13, Issue 2
By Cathy Kristiansen
NIH and its partners are pursuing creative new ways to move biomedical innovations more quickly from publicly-funded laboratories to developers to factories. Their goal is to ensure new products such as vaccines and more affordable HIV/AIDS treatments reach those who need them most in low-resource countries.
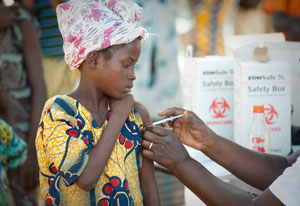
Photo by Gabe Bienczycki/PATH
NIH and partners are finding creative ways to steer new
technologies from invention to development to delivery of
vaccines and drugs to low-resource countries.
"We want to have an impact where we can, in areas of the world where the disease burden is heavy," said Steven Ferguson, Deputy Director of Licensing and Entrepreneurship in NIH's Office of Technology Transfer (OTT). "Diseases know no national boundaries and with the globalization of living styles, we just have to have a global approach." The OTT was established in 1989 to centrally manage technological innovations made by intramural scientists at the NIH and the FDA, including the licensing, patents and royalties.
Three key changes jolted the status quo in how biomedical inventions are handled: new U.S. technology transfer legislation, more favorable licensing agreement terms and the rise of global partnerships to steer products through development to low-resource markets.
Developing a new compound usually costs hundreds of millions of dollars and is fraught with risk, Ferguson noted. He compared the typical path for an innovation born at a U.S. government laboratory to reach the bodies of humans with the three-stage launch of a rocket. In the initial stage, scientists come up with a new compound or technique, such as a chemical that kills the parasite responsible for river blindness or a protein that induces a strong immune response to an influenza virus, and they test it for safety in animals or even in a few dozen people.
In the second stage, a biotechnology company - often a startup or pharmaceutical firm - acquires permission to make its own products using the technology. It conducts development often up to phase 2 clinical trials in perhaps 200 people to test for efficacy and side effects. Although NIH intramural scientists may not commercialize products themselves due to conflict of interest rules, they may help others with the basic or clinical research as part of their official duties. NIH-funded recipients outside government, such as universities, own the technology they invent and may develop it themselves or transfer it.
Finally, the biotech company - or an interested pharmaceutical firm that acquires the technology rights, sometimes by purchasing the smaller company - conducts large phase 3 trials involving 1,000 to 3,000 people. These are costly and must demonstrate that the product is safe and improves health in order to garner regulatory approval.
"There's developmental risk, financial risk and regulatory issues, particularly on novel approaches," Ferguson said. Against all this, the developing and emerging country markets might be small and the price charged must be low enough to be affordable, he noted. "The risk, on top of something that perhaps doesn't have a very strong market in Western countries, makes for a difficult overall proposition for development."
But more economical biotechnology products are now finding their way to low-resource settings. "We are doing more and more licenses at non-U.S. companies," Ferguson said. "It is a relatively small number of agreements, about 300 so far including those completed in non-U.S. Western countries, but they have a huge health impact. This will make a real difference from a global health perspective, especially for the several dozen to date, done directly in emerging or developing countries."

Photo courtesy of NIAID
New laws, licensing terms and global partnerships are
speeding translation of scientific discoveries into lifesaving
biomedical products.
The trigger for a proactive approach to technology transfer at NIH was a change in U.S. legislation. In the past, the government retained the rights to all technology produced by both NIH and NIH-funded scientists. But rather than attracting developers to make products and share the revenue with the government, most technology gathered proverbial dust on the shelf. So Congress passed legislation in 1980 to require the formation of formal technology transfer offices and programs at both federal labs like at NIH and at government-funded institutions. The Bayh-Dole Act allows extramural government-funded grantees to hold the rights to their innovations and the similar Stevenson-Wydler Act enables agencies to own and manage their intramural innovations.
But even with more favorable conditions for transferring ideas out for development, a number of new products were still beyond the reach of many country budgets. So in recent years, NIH and other public and private organizations have partnered to bridge this gap, negotiating more favorable licensing and manufacturing agreements, raising funds to purchase vaccines and drugs, and securing government collaboration in developing countries.
This has triggered a surge in new products using technologies licensed by the OTT - more than 600 across all markets to date. One is the meningitis A vaccine. The NIH OTT licensed the vaccine conjugation technology developed intramurally at the FDA to the nonprofit PATH, which teamed up with the WHO to set in motion production of the vaccine at a prearranged price. In another case, OTT transferred vaccine technology to South Korea's International Vaccine Institute for a typhoid vaccine produced in Indonesia and India for Asian populations. Also, the nonprofit GAVI Alliance was formed to bring low-cost vaccines to global populations via negotiations on technology transfer, manufacture and sale of these products. It garners funding from international organizations, donor governments, philanthropic groups and the pharmaceutical industry.
Photo courtesy of NIAID
Special agreements by the President's Emergency Plan for
AIDS Relief and nonprofits such as GAVI allow low-cost drugs
for HIV/AIDS and vaccines for many diseases to reach
low-resource countries, even while patent protection
continues in high-income countries.
In another approach supported by the President's Emergency Plan for AIDS Relief (PEPFAR), the FDA introduced a special licensing agreement for generic antiretrovirals to be produced and sold in developing countries, while upholding the brand-name drug's patent protection at home. These cheaper drugs mean millions more patients can access lifesaving treatments.
The changing face of technology transfer carries several risks from a corporate perspective, not least in opening up an alternative supply chain of drugs that could filter back into developed countries and threaten sales of pharmaceutical companies there, Ferguson noted. And enforcement of intellectual property rights is weak in some countries, eroding the willingness of a company to pour money into drug development if their findings might be snatched by another firm. But, he said, "That's all been changing."
For its part, NIH's OTT and technology transfer staff in the Institutes and Centers are raising global awareness of intellectual property and technology transfer issues by sending trainers as part of NIH or other federal agency programs to a number of low- and middle-income countries. In addition, the OTT offers a mentoring program in technology transfer on the NIH campus for officials from foreign research institutes or government agencies.
The OTT currently holds rights to nearly 8,000 inventions and has licensed technology relevant to diseases such as HIV/AIDS, malaria, dengue, rotavirus, meningitis and typhoid fever. License negotiations are completed or ongoing with a growing number of public and private institutions in India, Mexico, Brazil, China, Korea, Egypt, Argentina and South Africa.
"We are finding a home for the technology where the real need is, where it can have a large public health impact," Ferguson noted. "It has been really exciting to see this level of interest and activity, where 10 years ago we had almost none."
Global health vaccines using NIH's OTT technology
NIH's Office of Transfer Technology has granted numerous licenses for compounds with potential in global health, for instance technology for vaccines against dengue hemorrhagic fever, Ebola and Marburg viruses, rotavirus, HIV, human parvovirus B19, malaria and Japanese encephalitis potential and treatments against vascular conditions brought on by sickle cell disease, glaucoma and tuberculosis. The following examples show where some products are being developed and sold.
- Vaccine target: Meningitis A
- Licensee's country: India, Mexico, South Africa
- Distribution region: Sub-Saharan Africa, Middle East, Asia, Latin America, Caribbean
- Vaccine target: Rotavirus
- Licensee's country: Brazil, China, India, U.S.
- Distribution region: Latin America, Caribbean, Asia, Africa, Middle East
- Vaccine target: Typhoid fever
- Licensee's country: Indonesia, India
- Distribution region: Southeast Asia
- Vaccine target: Dengue
- Licensee's country: Brazil, India, U.S.
- Distribution region: Latin America, Caribbean, Asia
- Vaccine target: Varicella
- Licensee's country: Egypt
- Distribution region: Africa, Middle East
More Information
More Technology Transfer Resources
- Additional training tools
- Organizations engaged in tech transfer:
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.