NIH ClinRegs database provides international clinical trial regulations
May / June 2019 | Volume 18, Number 3
NIH has expanded
ClinRegs, its free, online database of international clinical trial regulations to include information from 20 countries. The site also now offers real-time updates of regulatory changes to its email subscribers.
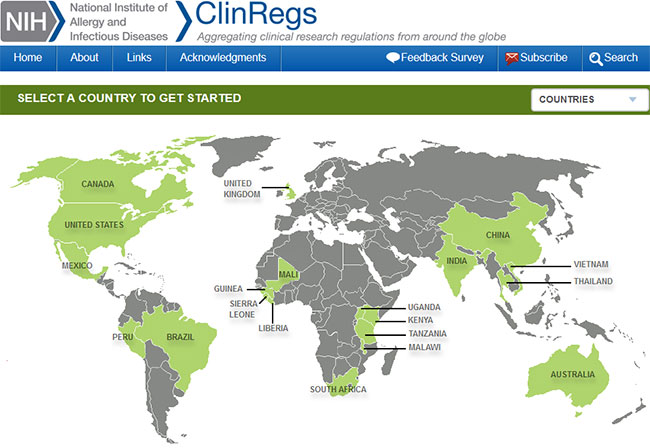
The NIH
ClinRegs database of clinical trial regulations contains information for 20 countries, shown in green.
It was launched in 2014 by the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). Since then, it has served more than 68,000 users from 157 countries. The resource includes listings of regulatory authority, ethics committee, clinical trial lifecycle and sponsorship. It also provides regulatory authority contact information, and estimated application review and approval times.
Each country regulatory profile contains:
- Concise, comprehensive summaries of country requirements written in English
- Quick facts table for answers to frequently asked questions
- Direct links to regulations on the country’s regulatory authority websites
- A country comparison feature for displaying the regulations for two countries side-by-side
Users can also reach the ClinRegs team directly by clicking the Contact Us button on the top right of the website.
More Information
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.