Focus: Fogarty grantees publish book to boost biomedical engineering in Africa
September / October 2019 | Volume 18, Number 5
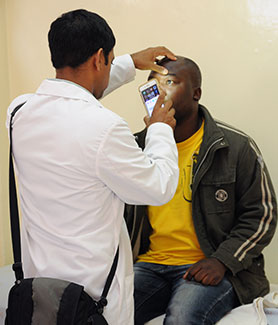
Photo courtesy of Dr. Omar Siddiqi
Biomedical engineers are needed in Africa to develop
locally relevant technologies and applications to
improve health.
Africa’s unique context and health care challenges would benefit from specially tailored technology solutions, rather than its current reliance on imports from industrialized nations. Appropriately designed technologies can be used in the prevention, diagnosis, monitoring and treatment of disease, which can save lives as well as money. To meet that need, the continent must develop its own biomedical engineers who are familiar with local needs and resource constraints, according to
Biomedical Engineering for Africa, a book published recently by Fogarty grantees and collaborators.
“The field of biomedical engineering is central to health technology innovation,” the authors suggested. “Transplanting healthcare systems and technologies from high-income countries to resource-limited settings is slow and expensive, and in many instances, challenging if not impossible.” Developing new healthcare delivery methods and devices tailored to LMIC environments and conditions is a faster, better and more cost-effective alternative, they said.
Grounded in the African context, the 22-chapter open-access volume is intended as a resource for practitioners, academics, students and university leaders planning to establish new biomedical engineering programs. Although focused on Africa, the publication is relevant to other low- and middle-income country (LMIC) settings and provides insights to guide global health initiatives focused on technology innovation.
The book was produced with support from Fogarty and Northwestern University (NU) in Chicago, and was published by the University of Cape Town (UCT) Libraries. Nearly 90 authors from NU and across Africa contributed to the volume, which was edited by Dr. Tania S. Douglas, a biomedical engineering professor at UCT. A
Fogarty Framework Innovation grant to NU helped establish departments of biomedical engineering at the Universities of Ibadan and Lagos in Nigeria, and facilitated collaboration with biomedical engineers at UCT.
In Africa, economic growth, increasing healthcare expenditure, the availability of digital technologies, and young populations provide opportunities for the development of robust health technology innovation systems, according to the book’s authors. For local innovation to become the norm across the continent, capabilities must be developed to conduct needs assessments, market analyses, product design, prototyping and testing, manufacturing, distribution and management. To produce products that are actually used, biomedical engineers must develop an in-depth understanding of the social factors that impact technology adoption.
Stronger linkage with clinicians needed
Creating technologies suitable for LMICs is not the only engineering need. More than half of medical equipment in developing countries is defective, according to WHO estimates, which causes delays in diagnosis and treatment, higher costs and sometimes loss of life. One book chapter makes the case for closer collaboration between engineers and clinicians to improve maintenance capability, as well as advance engineers’ understanding of the technologies needed to improve patient care. For instance, in hospitals lacking incubators, engineers could develop low-cost baby warmers to reduce newborn deaths. However, identifying the problem is only the first step. A needs assessment should be performed, the authors suggested, noting the WHO recommends customization of the process according to the specific circumstances.
Best practices might include:
- Having both groups of personnel agree on the unified goal of establishing good patient outcomes.
- Fostering mutual understanding and respect for the unique roles and skills each group brings in working towards achieving the common goal.
- Ensuring good communication in problem-solving activities related to patient care.
- Raising awareness with hospital management and health system administrators of the benefits of clinician-biomedical engineer cooperation in order to garner their support.
Africa is ripe for entrepreneurship
Africa has received global attention in the 21st century as the continent of growth and opportunities. One of the book’s chapters explores its current climate for innovation, noting there has been considerable debate on how African governments can create jobs and develop home-grown business leaders able to access global markets and propel growth. That is partly dependent on the capacity and willingness of African countries to create an environment conducive to the emergence of entrepreneurship in the public and private sectors, the authors said.
There have already been a number of successful products developed by Africans, for use in Africa, which are described in the text. The Cardio-Pad, created by Cameroonian Arthur Zang, is used to detect cardiovascular disease in patients who live in remote areas by providing wireless data transmission to a cardiologist. Deaftronics, a company in Botswana, built a prototype charger for solar power hearing aid batteries that last for three years and can be used with many hearing aids available on the market. It was developed for the hearing impaired in developing countries who are unable to access electricity. In South Africa, Power Free Education Technology produced a wind-up Doppler ultrasound fetal heart rate monitor to detect fetal distress for use in rural areas.
The book contains several chapters detailing the process used to develop technologies in Africa including an innovative approach to improve burn wound treatment, an infant warming device and a needle disposal machine.
Future successes will depend, in part, on the “robustness” of higher education to train and develop biomedical engineers, the authors said. “The challenge in developing an African entrepreneurial biomedical engineering environment is not only the lack of infrastructure but also the lack of innovative capacity and ecosystems that support innovation, as well as a fragile healthcare system on the continent.”
There are few biomedical engineering (BME) programs in Africa prepared to meet these needs, the publication noted. In 2008, only 12 universities in six African countries offered BME programs, compared with nearly 230 universities in North America. In 2012, a group of African universities founded the African Biomedical Engineering Consortium (ABEC) with the goal of increasing engineering capacity and nurturing entrepreneurial and innovative skills across the continent. It has developed a standard undergraduate BME curriculum that has been adopted by some of the participating universities.
Developing frugal design for low-resource settings
Frugal Biodesign Approach Stages
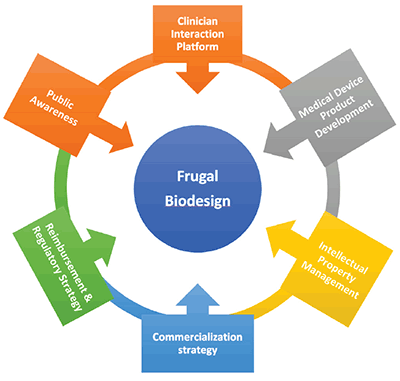
Image from Biomedical Engineering in Africa
Frugal Biodesign flow chart description: Circle in center labeled Frugal
Biodesign surrounded by 6 boxes with arrows pointing to the center
labeled clockwise from the top: Clinical Interaction Platform; Medical Device
Product Development; Intellectual Property Management; Commercialization
Strategy; Reimbursement & Regulatory Strategy; Public Awareness.
Frugal Biodesign - a unique approach to medical device design that is suited specifically to developing countries - is examined in one book chapter. The course, developed at UCT in South Africa, is aimed at stimulating postgraduate students studying BME to devise inexpensive and, more importantly, innovative solutions to medical problems. It recognizes the limitations that South Africa and other developing countries experience in terms of human, financial and physical resources. The medical devices that the students work on during this course are informed by clinicians. The course adopts a cyclical and dynamic approach that involves the constant exchange of information between multiple stakeholders.
The two-semester, 10-month curriculum is intended to fast-track the process of ideation, which begins with identifying a need and continues until proof of concept is achieved. The course prepares students to develop medical devices that are appropriate to needs, at low cost. It takes into consideration the constraints which hinder technological development in low-income settings such as lack of funding, skilled personnel and infrastructure. By leveraging the talents of a pool of students drawn from different engineering backgrounds and the insights from clinical partners, an interdisciplinary approach is applied to the problems being addressed.
One UCT success has been the adoption of 3D printing to produce medical devices, such as a crutch that attaches to spectacles to elevate the upper eyelid in patients with
myasthenia gravis, a condition that can cause eyelids to droop and for which treatment options are limited in low-resource settings.
UCT released the design as an open source innovation that can be downloaded at no cost.

Photo from Biomedical Engineering in Africa
A 3D-printed device attaches to spectacles to elevate the
upper eyelid in patients with
myasthenia gravis, a condition
for which treatment options are limited in low-resource
settings.
Mobile health is powerful platform for healthcare
With the ubiquity of cellphones in much of Africa, mobile health holds tremendous potential for extending healthcare services to remote areas and poor people in Africa. One section of the text is devoted to exploring these opportunities. Many disease outbreaks in Africa have been caused by lack of, or late, reporting. While most countries have implemented successful surveillance systems that include training for healthcare workers, many are still paper-based. The high penetration rates and increasing adoption of mobile devices in Africa has the potential to enhance the development of effective disease surveillance systems.
The main drivers for mHealth in Africa include high mobile penetration, increasing cellphone subscriptions and the high burden of disease, the authors said. There are opportunities for mHealth projects to provide health education and create awareness, deliver personalized and remote patient monitoring, conduct disease surveillance and build clinical decision support systems. Possible challenges include low health literacy levels; poor network, power supply and hospital infrastructures; socio-cultural barriers; and lack of political will by governments to support mHealth projects. To build scalable and sustainable mHealth systems, there is need to foster strong public-private partnerships, develop mHealth systems that can be easily integrated into existing healthcare systems, and produce health information systems that ensure the security, integrity and privacy of patient data.
Incorporating bioethics into innovation
Biomedical engineering is unique as an engineering specialty, in that it is a synthesis of engineering principles and medical practice, the authors noted. The unique professional identity of biomedical engineering requires ethical frameworks that are sensitive to both medical and engineering standards. While engineering ethics is narrowly focused on safety, and medical ethics on patient care, biomedical engineering ethics is at the intersection of safety and patient care, beginning at scientific experimentation and design, and extending through medical practice and administration. Understanding the history of engineering ethics and biomedical ethics is essential to understanding the evolution and future of modern biomedical engineering ethics, according to the authors.
Protecting intellectual property
Developing novel biomedical products including drugs, devices, assays and equipment is a lengthy, and possibly expensive, process that starts with an innovative idea that potentially meets a critical medical need. Intellectual property protection may be needed to prevent the concept from being used or even marketed by another party, the authors said in a chapter devoted to the topic. Trademarks, patents, copyright and trade secrets are some issues that may be considered. Commercialization paths such as licensing and establishing spin-off companies may also be relevant. In addition, biomedical engineers must obtain regulatory approval to bring their products to market. After proving the safety and efficacy of their invention, they then will need to convince clinicians to adopt it, which can be time consuming.
Research universities may play a role in the process, ranging from educating the innovators to developing prototypes. Universities may even assist in licensing and start-up activities. A robust development pipeline involves identification and protection of the intellectual property and a clear, well-defined partnership between the institution and its inventors.
Regulating medical devices in Africa

Photo by Susan Urmy/Vanderbilt University
Tiny magnets and plastic tubing are being developed by
Fogarty grantees to provide a low-cost method of
diagnosing malaria.
The book also contains an overview of medical device regulation in 10 African countries, noting they have a strong focus on imports, which is not surprising given their heavy reliance on medical devices from developed countries. For example, South Africa, Nigeria and Egypt - considered to be the largest markets and economies in Africa - continue to be dominated by the supply of orthopedics, prosthetics, patient aids and consumables from the U.S. The regulatory approval process for medical devices in Africa is lengthy, not transparent and skewed toward controlling entry into the market of substandard imports that pose a health risk, the authors reported. None of the ten countries discussed has specific regulations or regulatory bodies dedicated solely to medical devices, which can cause difficulties and delays in the process.
However, medical device regulations in Africa are designed along the framework of models used in developed countries. For example, the requirements for importation and exportation of medical devices in South Africa, Algeria, Kenya and Ethiopia are similar to the internationally recognized regulatory programs in Europe, the U.S. and Australia. This is important in that it aligns African countries with a harmonized framework for medical device regulation. In an era of globalization, this facilitates cooperation among regulators and the industry.
Beyond regulations, much could be done to promote the development of medical device industries in African countries. In Ethiopia, Ghana, Kenya and Tanzania, a WHO framework for local production and access to essential medical products is being implemented to stimulate innovation and provide technical assistance. National policymakers can also establish preferential procurement of domestically manufactured medical devices to increase demand for home-grown products.
“African governments can play a leading role in encouraging the development of their domestic medical device industries,” the authors said, “not only by establishing medical device regulations and providing adequate resources for their implementation, but also through broader policy considerations.”
More Information
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.