Research offers new hope to combat HIV/AIDS
August 2010 | Volume 9, Issue 4
Research presented at this year’s International AIDS conference in Vienna offered new hope to those working on HIV/AIDS treatment and prevention. Fogarty grantees and trainees presented promising developments on four topics that generated keen interest: a vaginal gel that reduces the risk of HIV transmission, proof breastfeeding is safe when either the mother or baby is on antiretrovirals, and new recommendations on when treatment should begin.
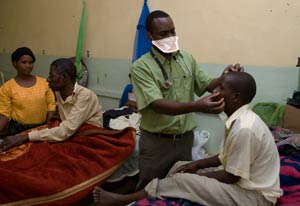
Photo by Richard Lord
At the recent international AIDS conference, Fogarty-
supported scientists presented several research
advances, including new treatment recommendations
for patients co-infected with HIV and TB, like this
one in Malawi.
Microbicide gel reduces HIV transmission
Some scientists are predicting a new vaginal microbicide will be a “game changer,” giving women a new effective tool to help stop transmission. Studies show a gel containing tenofovir has been found to reduce the chance of contracting HIV by 39 percent and has no notable side effects.
Women and girls make up the majority of the newly HIV-infected in sub-Saharan Africa. The gel would provide discreet protection they can control, even if they can’t guarantee their partner’s sexual fidelity or condom use.
The results showed women who used the gel most regularly reduced their chances of infection by 54 percent, according to a study by the Center for the AIDS Program of Research in South Africa (CAPRISA). The trial was led by Dr. Quarraisha Abdool Karim, with participation from eight scientists who have been trained through Fogarty’s AIDS International Training and Research Program. A Fogarty bioethics grant also provided expertise.
More testing is needed before the gel is made widely available, the researchers say. Its cost is estimated to be less than 25 cents per application. USAID funded the trial.
Guidelines change for antiretroviral therapy
There are advantages to starting HIV treatment earlier than previously thought, new studies show. Researchers are recommending drug therapy begin once a patient's CD4 level has fallen to 350, when they are still relatively healthy.
The current standard has been that treatment should start when the CD4 level is below 200. At that stage most patients have some symptoms of the disease.
Accordingly, the U.N. is lowering its threshold for treating HIV-positive individuals in developing countries in the hope that earlier treatment will prevent hospitalizations and reduce secondary medical costs. Patients whose infections are under control are much less likely to transmit the virus.
Guidelines are also changing for patients co-infected with HIV and TB. This population benefits significantly by having HIV therapy start two weeks after beginning TB treatment, rather than waiting the standard eight weeks. Beginning HIV therapy before the TB infection is under control may cause an immune system reaction that can worsen the patient’s condition and even cause death.
The findings are a result of a clinical trial in Cambodia, co-funded by the National Institute of Allergy and Infectious Diseases and supported by Fogarty trainees.
Breastfeeding proven safe with ARTs
It is safe for HIV-positive mothers to breastfeed their babies as long as one or the other is on antiretrovirals during the nursing period, as shown by two recent studies supported by Fogarty trainees.
In a trial conducted in Botswana, pregnant women in the third trimester were started on antiretroviral therapy that extended six months into the nursing period. The infants received a single dose of one type of drug and four weeks of another antriretroviral, resulting in a 1.1 percent rate of mother-to-child transmission, the lowest ever reported in Africa.
Researchers in Malawi tested whether treating mothers or infants during breastfeeding led to better outcomes. Their infant-treatment trial reduced the transmission rate to 1.7 percent, while the transmission rate when mothers were treated was 2.9 percent.
Journal references
Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Karim QA, Karim SS, Frohlich JA,and others on behalf of the CAPRISA 004 Trial Group. Science, 2010 Jul 19.
Early versus Standard Antiretroviral Therapy for HIV-Infected Adults in Haiti. Severe P, Juste MAJ, Ambroise A, Eliacin L, Marchand C, Apollon S, Edwards A, Bang H, Nicotera J, Godfrey C, Gulick RM, Johnson WD, Jr., Pape JW, Fitzgerald DW. N. Engl. J. Med. 2010; 363: 257-265.
Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per μL in Europe and North America: a pooled cohort observational study. The Lancet 2010, Volume 376, Issue 9738, 340-345.
Antiretroviral Regimens in Pregnancy and Breast-Feeding in Botswana. R.L. Shapiro, M.D., M.P.H., M.D. Hughes, Ph.D., A. Ogwu, M.B., B.S., C. Moffat, M.B., Ch. B., M.P.H., I. Thior, M.D., O. Jayeoba, M.B., Ch.B., and others. N Engl J Med 2010; 362:2282-2294. Online June 17, 2010.
Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. Charles S. Chasela, Ph.D., Michael G. Hudgens, Ph.D., Denise J. Jamieson, M.D., M.P.H., Dumbani Kayira, M.B., B.S., Mina C. Hosseinipour, M.D., M.P.H., Charles M. van der Horst, M.D. and others. N Engl J Med 2010; 362:2271-2281. Online June 17, 2010.
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.